斜四金刚烷的立体选择性全合成
近日,瑞士巴塞尔大学Christof Sparr团队研究了斜四金刚烷的立体选择性全合成。该研究于2026年1月6日发表在《自然-化学》杂志上。
金刚石的基本手性组分——斜四金刚烷——具有极高的刚性、稳定性和精确定义的几何结构,是σ-螺烯母体结构的缩影。虽然斜四金刚烷在化石燃料中天然存在,但其微量含量极低,几十年来人们对其选择性合成的努力仍未取得成果。
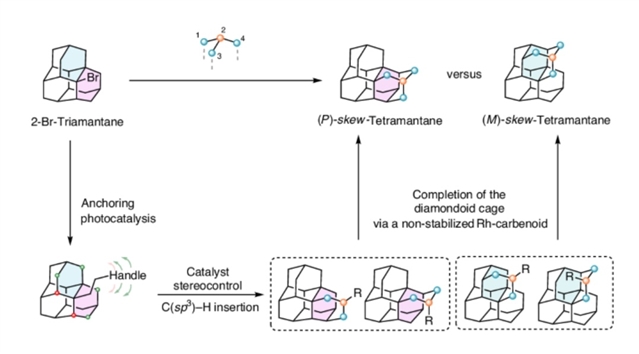
随着光催化和过渡金属催化在驯服自由基和卡宾物种方面的最新进展,研究组现已设计出一种通过立体选择性金刚烷笼扩展来实现斜四金刚烷的靶向全合成方法。首先,通过光催化Giese反应实现帽盖连接,同时利用Davies的手性铑催化剂通过分子内C(sp3)−H插入实现显著的区域、非对映体和对映体控制。
经过Buchner–Curtius–Schlotterbeck环扩展和立体选择性的Mukaiyama水合反应后,通过非稳定卡宾的分子内C(sp3)−H插入完成与金刚烷斜四金刚烷结构的融合。研究组表明,该方法可获得由催化剂定义σ-螺烯性的异构纯形式的合成斜四金刚烷,标志着向更高阶金刚烷类化合物选择性途径的迈进。
附:英文原文
Title: Stereoselective total synthesis of skew-tetramantane
Author: Li, Xiao-Yu, Sparr, Christof
Issue&Volume: 2026-01-06
Abstract: Diamond’s elementary chiral constituent—skew-tetramantane—features extreme rigidity, stability and a precisely defined geometry, epitomizing the parent structure of a σ-helicene. While skew-tetramantane is naturally occurring in trace fractions in fossil fuels, efforts over several decades towards its selective synthesis remained unfruitful. With the recent advances in photocatalysis and transition metal catalysis to tame radical and carbene species, we have now devised a targeted total synthesis of skew-tetramantane by means of a stereoselective adamantalogous cage extension. A first cap attachment was effected by a photocatalytic Giese reaction, while remarkable regio-, diastereo- and enantiocontrol were achieved by an intramolecular C(sp3)H insertion using Davies’ chiral rhodium catalysts. After a Buchner–Curtius–Schlotterbeck ring expansion and a stereoselective Mukaiyama hydration, the fusion to the adamantine skew-tetramantane structure was completed by an intramolecular C(sp3)H insertion of a non-stabilized carbenoid. Here we show that this approach provides access to synthetic skew-tetramantane in isomerically pure form with σ-helicity defined by the catalyst, marking a selective pathway to higher diamondoids.
DOI: 10.1038/s41557-025-02026-0
Source: https://www.nature.com/articles/s41557-025-02026-0
